What are different phases of clinical trials and how to design and analyze?
In-Brief
- Biostatistics in health care used to analyze and also help to solve the problem faced by public healthcare-related and biomedical, clinical and population-based research.
- Clinical trial statistical analysis is the collection and interpretation of data to uncover patterns and trends. It is an element of data analytics. In the context of business intelligence (BI), the statistical analysis includes collecting and analysis of data smples.
Introduction
Preclinical biostatistics for health professionals in a lab can provide foundation data about how a drug may work and its satisfaction. It is not a substitute for analysis that shows how the drug will collaborate with the human body. The word “clinical trials” or “clinical research” denotes a study that has been conducted in people. Researchers conduct clinical trials to answer particular research questions related to a drug candidate. The clinical test follows a difficult series from early, small-scale, phase I analyze to a large-stage, large scale, and phase III studies. If treatment is successful in one phase, it moves on to the next phase.
An effective clinical trial process lasts until the developer produces a marketing application with the U.S Food and Drug Administration (FDA) or regulatory assistance in another country for the medication to be permitted for doctors to recommend to people. Clinical biostatistics services can help identify the best way to deploy resources to treat populations. To control the epidemic, the goal is not only finding the best way to treat an infected person but also to control the spread in the population.
Though the stages and design of clinical trials may vary from specific sicknesses and specialized medicines, such as cancer drugs or gene treatments, here is a general overview of each phase of a clinical trial for most medications:
Phase 0
It is the primary clinical trial that has been done among people; the main objective is to learn and understand how a drug is processed in the body and how it affects the human body. In this trial test, a very small drug dose will be given to 10 to 15 people.
Phase I
The objective of Phase I is to determine the best dose among new drug sample with the fewest side effects. The sample will be tested in a group of 15 to 30 people. Doctors start by providing a very low dose of the drug to a few people. The higher dose is given to people only after the side effects are known, or the severity is known. The drug may benefit people, but phase I trials are mainly done to test for drug safety. Once the drug is recognized to be safe, then it will be promoted to the next phase of a clinical trial.
Phase I clinical trials are generally done among healthy volunteers, i.e. healthy people. Usually, 20 to 80 healthy volunteers participate in Phase I. It is used to measure how the drug works in the human body and the side effects associated with increased dosage.
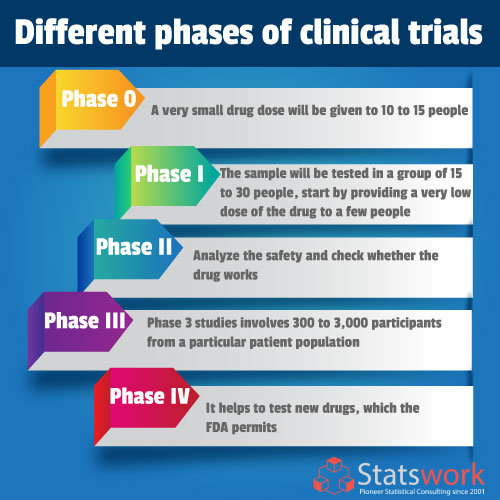
Phase II
Phase II trials further assess the safety and check whether the drug works. The drug is usually tried among patients with a particular type of cancer. Phase II trials are conducted among groups of people with a particular disorder compared to Phase I trials. Often, new drugs consolidation is tested among patients closely watched to identify whether the drug works properly. However, the new drug is rarely associated with the current drug (standard-of-care) that is already in use. If a drug works then, it can be tested in phase III clinical trial.
Ideally, the role of a statistician can be quite varied in Phase II clinical trial. Statisticians help translate the findings from Phase I into a Phase II design that aims to determine the dose and exposure range in which the drug is active.
Phase III
It helps in comparing a new drug to the standard-of-care drug. These trials calculate the side effects of each drug and which drug works better. Phase III trials enrol more than 100 patients. These trials are done among a random group of people, who are called trial arms if, by any chance, the patient in the particular group, i.e. within the same trial arm, are alike. Scientists will face difficulties in understanding whether the clinical trial results are due to the treatment, and it is not because of differences found among the groups. A computer program is used randomly among these trial arms. In Phases III, there will always be more than two treatment groups. The control group gets stand-of-care treatment. The other groups get the new treatment, but neither you nor your doctor can select your group. You will also not aware of which group you belong to until the trial is over.
All patients in a phase III analysis is watched closely. The analysis will be stopped soon if the new drugs’ side effects are severe or if one group has a better result. Phase III clinical trials are often necessary before the FDA approved the use of a new drug for the public. Phase 3 studies typically involve 300 to 3,000 participants from a particular patient population for which the medicine are ultimately intended to be used.
When one or more Phase 3 trials got over, the researchers inspect the outcome and select whether the drug has proven effectiveness and an adequate safety profile in treating a disease. If so, the organization can submit a New Drug Application (NDA), which comprises all of the facts and information collected at each stage of the process through the outcome of Phase 3 clinical trial(s) and other data needed by the relevant regulatory authority. The NDA is submitted to the FDA to get marketing approval (or analogous applications may be surrender to other regulatory agencies outside). Because Phase 3 trial results often offer fundamental approval, sometimes they are also called pivotal trials. If the drug is accepted, doctors can prescribe the medication for their patients.
Phase IV
It helps to test new drugs, which the FDA permits. The drug is tested among several hundred or thousands of patients. It helps in short-lived and long-lasting side effects and safety identification among the research. Some rare cases, side effects may only be identified in large groups of people. Doctors can also learn more about how well the drug works and if it’s helpful when used with other treatments.
Conclusion
A clinical biostatistics service is essential to investigate drug or device development programmers to determine the efficacy and effectiveness of clinical trials. It includes the study designs, study conduct, determining the most effective Data collection points and how analysis and reporting should be performed.
References
- Whitehead, J. (1997). The design and analysis of sequential clinical trials. John Wiley & Sons.
- Peto, R., Pike, M., Armitage, P., Breslow, N. E., Cox, D. R., Howard, S. V., … & Smith, P. G. (1977). Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. British journal of cancer, 35(1), 1-39.



 Next Post
Next Post